What Is an Expression of Boyle's Law K Constant
Stokess Law by Dimensional Analysis. Stoke found that the viscous force F depends upon i coefficient of viscosityη ii terminal velocity v of the body and iii radius r of the spherical body.
Gases Properties Formula Laws Derivation Graph
The total momentum remains constant.

. The proportionality constant k is the rate constant of the reaction. The ideal gas law is the final and most useful expression of the gas laws because it ties the. N is the amount of substance.
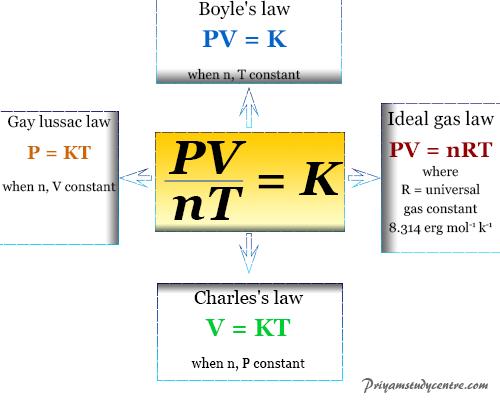
Boyles Law Gay-Lussacs Law Combined Gas Laws Grahams Law Ideal Gas Law. It is important to note that the expression of the rate law for a specific reaction can only be determined experimentally. P V n R T where R stands for ideal gas constant and equals 83144598 Jmol K.
Where P is the pressure. These specific relationships stem from Charless Law Boyles Law and Gay-Lussacs Law. R is the ideal gas constant.
Boyles law was put forward by the Anglo-Irish. Ideal Gas Law PV nRT 2. 3 Ideal Gas Law Ideal Gas Law PV nRT The pressure of a gas times its volume equals the number of moles of the gas times a constant R times the temperature of the gas.
Suppose for example that two particles interact. This fact known as the law of conservation of momentum is implied by Newtons laws of motion. Physics Grade XI Notes.
N is the amount of substance of the. During any process at least two of these properties change which can be compiled in combined gas law formula. As explained by the third law the forces between them are equal in magnitude but opposite in.
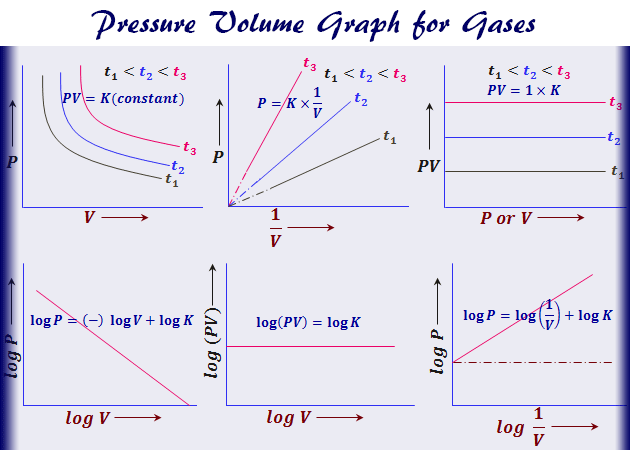
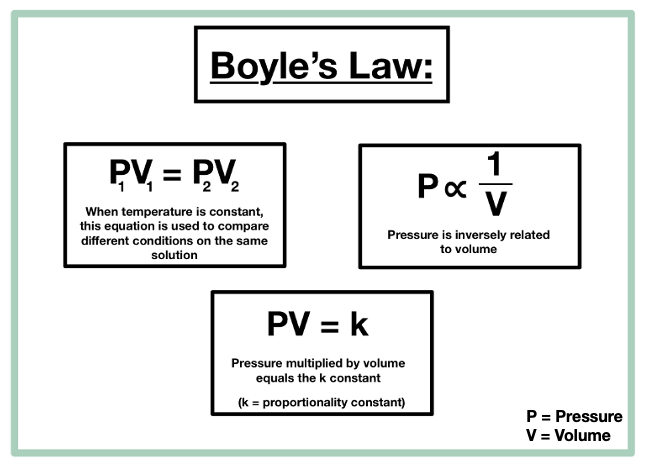
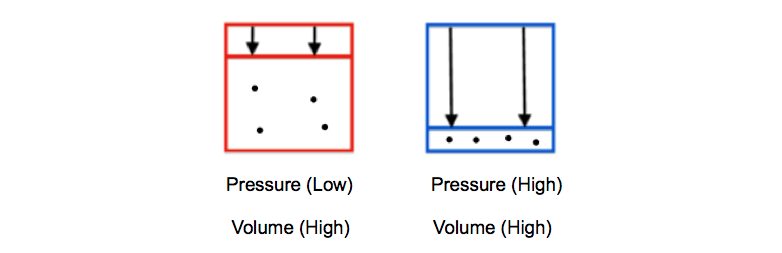
According to Boyles law for an ideal gas the pressure of the given mass is inversely proportional to its volume when the temperature is constant. Determination of Viscosity of Liquid by Using Stokess Law. The rate law expression cannot be obtained from the balanced chemical equation since the partial orders of the reactants are not necessarily equal to the stoichiometric coefficients.
It is an expression of one of the fundamental symmetries of space and time. The Ideal Gas Law is a simple equation demonstrating the relationship between temperature pressure and volume for gases. An ideal gas can be described by several parameters which are pressure p volume V temperature T and the amount of particles nThey are correlated with the equation.
R universal gas constant 83145 Jmol K N number of molecules k Boltzmann constant 138066 x 10-23 JK 8617385 x 10-5 eVK k RN A. In other words the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. PVnRT in which Ppressure VVolume nmoles of substance Rgas constant and TTemperature.
But there is also a. Where P is the pressure. It implies that.
Boyles law is a gas law which states that the pressure exerted by a gas of a given mass kept at a constant temperature is inversely proportional to the volume occupied by it. N A Avogadros number 60221 x 10 23 mol The ideal gas law can be viewed as arising from the kinetic pressure of gas molecules colliding with the walls of a container in accordance with Newtons laws. In short the ideal gas law states that the product of one gram molecules pressure and volume is equal to the product of the gass absolute temperature and the universal gas constant.
V is the volume. Ideal Gas Equation Units. Charless Law identifies the direct proportionality between volume and temperature at constant pressure Boyles Law identifies the inverse.
Relationships between Boyles Charless Gay-Lussacs Avogadros combined and ideal gas laws with the Boltzmann constant k B R N A n R N in each law properties circled are variable and properties not circled are held constant The ideal gas law is the equation of state for an ideal gas given by. Boyles Law states that the product of the volume and pressure for an ideal gas remains constant. The ideal gas law describes the property of a hypothetical ideal gas.
V is the volume. PV k Here k is a constant P is the pressure and V is the volume.

Boyel S Law Graph Chemistrygod

Boyle S Law Definition Equation Facts With Examples
Boyle S Law Clippard Knowledgebase
Gases Properties Formula Laws Derivation Graph

Boyle S Law Derivation Statement Formula Examples And Experiment State Of Matter Chemistry Youtube

Calculus 1 Derivatives And Related Rates 17 Of 24 Boyle S Law Dv Dt Youtube

Boyle S Law Pressure And Volume Youtube

Ideal Gas Equation And Absolute Temperature Boyle S Law Derivation
Boyle S Law Overview Formula Expii

Boyle S Law Definition Equation Facts With Examples

What Are The Gas Laws And Their Formulas By Chemistry Topics Learning Chemistry Online Medium

Boyle S Law Definition Equation Facts Britannica

Boyle S Law Overview Formula Expii

Boyle S Law Equation Or Formula Chemistrygod

A State Boyles Law B Give Its Lido

Boyle S Law Practice Problems Youtube

Boyle S Law Definition Equation Facts With Examples

Comments
Post a Comment